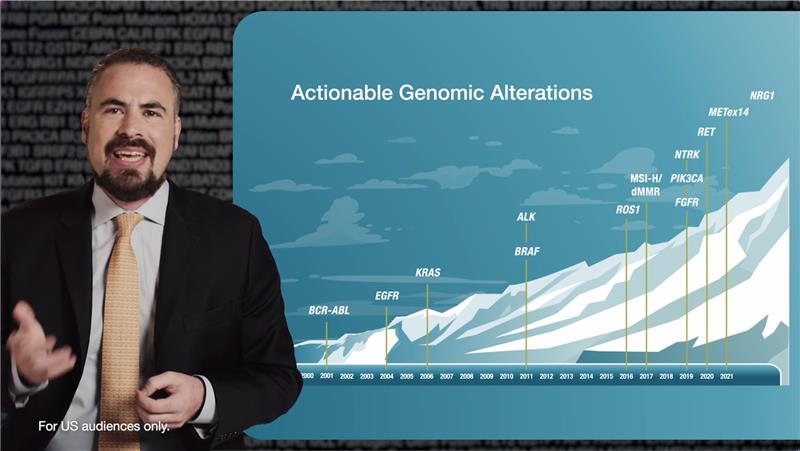
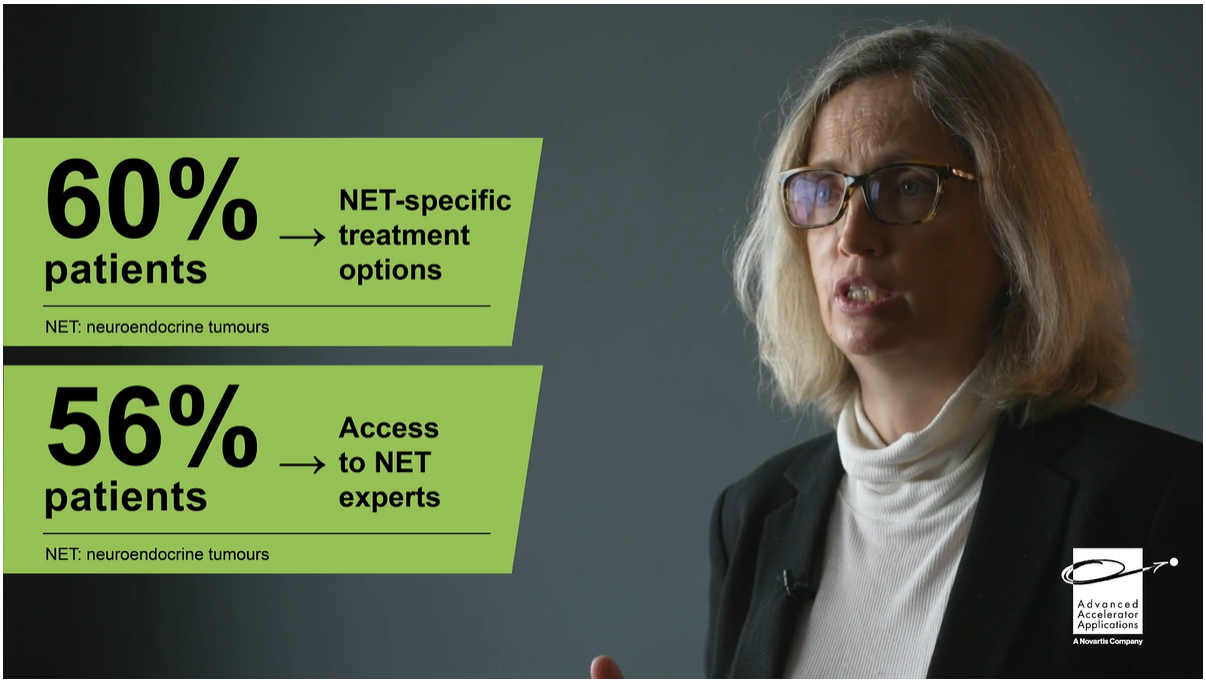
Learning Zone
View medical education and communication on key topics in the area of Oncology.
The Learning Zone is sponsored by Amgen Inc. and Sanofi Genzyme US. Neither party is involved with, or influences, the development of content

All Activities
View all Learning Zone activities

Content in this section is sponsored by the pharmaceutical industry and is developed by Touch Medical Communications (TMC) for touchONCOLOGY.
TMC’s mission is to provide HCPs with key education and communication through our unique Touch platform which combines clinical expertise with unparalleled society partnerships, long-term expert relationships and the Touch journal heritage, ultimately leading to fast implementation of positive clinical practice changes and improved patient outcomes. TMC’s "learner first" approach means that programming is delivered in multiple formats and can be accessed via multiple platforms, including mobile, allowing today’s on-the-go healthcare professionals to learn in the way that best suits them.